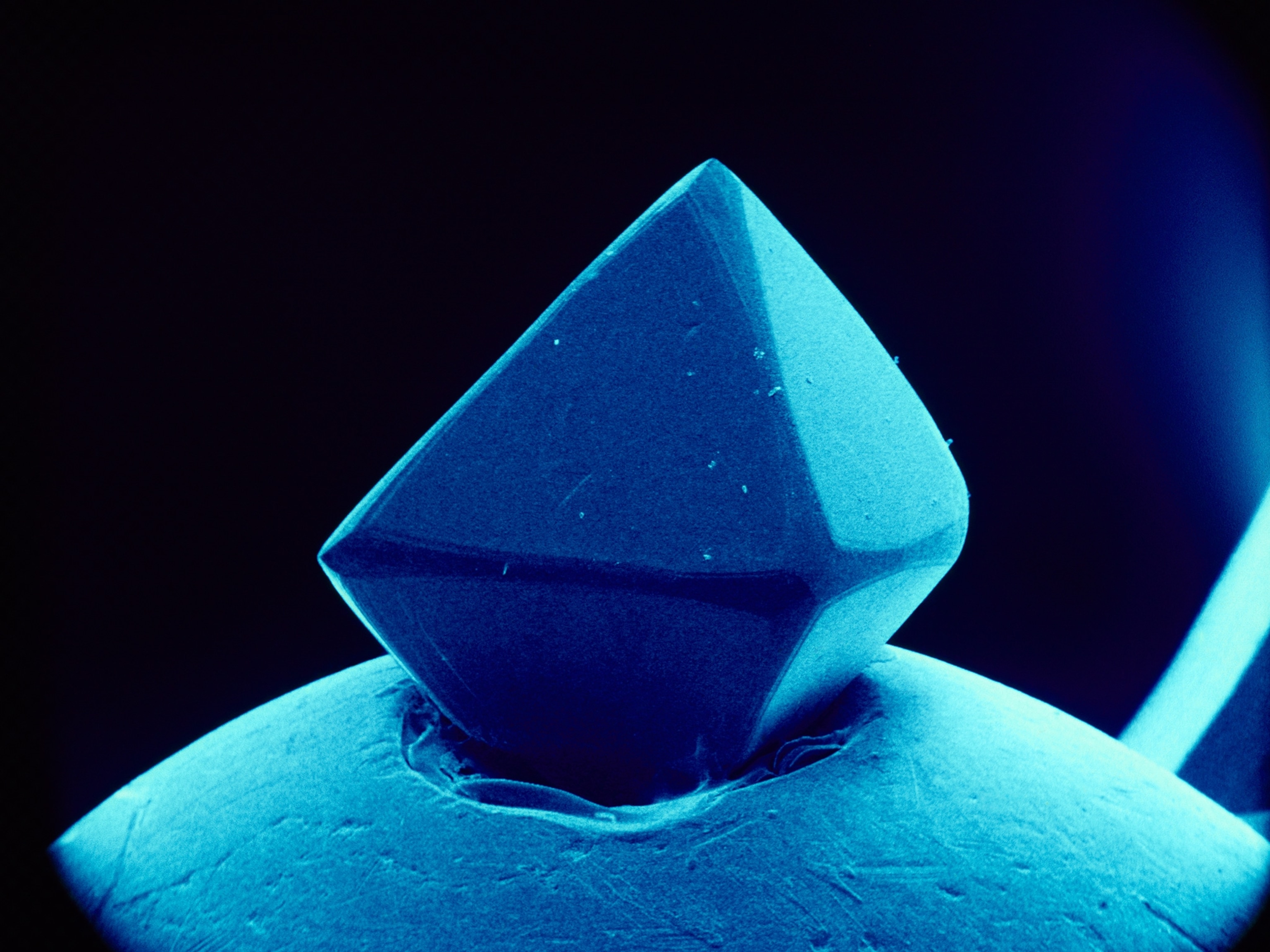
Michael Förster was disappointed. As a Ph.D. student at Australia's Macquarie University, Förster had spent months cooking rocks in an attempt to grow the flaky, shimmery mineral mica in the lab, and he had little to show for his efforts. But in a meeting with his supervisor, his dismay quickly turned to delight. Instead of unlocking the mysteries of micas, he realized, the work could explain the enigmatic formation of another sparkly mineral: diamond.
These precious stones often have curiously salty impurities that scientists have long struggled to explain. The new research, published this week in the journal Science Advances, suggests that these inclusions are tiny time capsules of the sediments that once lingered in ancient oceans.
These sediments can get dragged down deep inside Earth thanks to the constant recycling of our planet's surface at what are known as subduction zones, regions of the planet where one tectonic plate dives beneath another. The new work re-creates the complex reactions that take place between 62 and 124 miles beneath the surface, as these sediments and the rocks of deep Earth stew at soaring temperatures. And the key to these reactions seem to be ocean sediments.
“I was totally excited,” says Förster, who is now a postdoc at Macquarie. “I realized how special it was.”
Thomas Stachel, a diamond scientist at the University of Alberta, notes that this mechanism may not hold for the more ancient diamonds that formed billions of years ago on early Earth, when our baby planet was much hotter. But for the younger diamonds, he says, “this definitely is a very good and interesting explanation.
“I’m not sure this is the final word,” he adds. “But they produced something that is a very good match by melting the sediments.”
Sparkly innards
Diamonds typically form around a hundred miles below Earth's surface, crystallizing in what are known as cratonic roots—regions of old, stiff mantle that prop up overlying continents. So far, the deepest anyone has drilled into the crust is just over 7.6 miles, so no one has been able to directly study what happens at these extreme depths.
We only get to enjoy diamonds because they're brought closer to the surface during rare volcanic eruptions that unearth deep molten rocks known as kimberlite magma. But the exact conditions that lead to their formation have long remained a mystery. (Learn more about the estimated quadrillion tons of diamonds that lurk deep inside Earth.)
Diamonds aren’t just pretty. They also tell us a lot about the Earth.Karen Smit, Gemological Institute of America
One particularly curious question is how the chemistry of tiny fluid inclusions within many diamonds, known as fibrous diamonds, came to be. These fluids hold an unusual excess of potassium salts relative to sodium salts, chemical proportions not known to linger deep in Earth.
A 2015 study published in Nature suggested that this salty fluid was the remnant of ancient ocean water that had altered the seafloor, becoming entrapped in the minerals of the crust and later released when the slab of subducting rock plunged into Earth. But this still couldn't explain the unusual excess of potassium in the diamond's salty slurries, Förster says.
Deep Earth in a pill
In the latest work, the researchers re-created the conditions that might be encountered deep underground in a tiny platinum capsule. This tin can-shaped container was lined with carbon and then filled with a layer of ground-up ocean floor sediments recovered from the International Ocean Discovery Project, along with a layer of ground-up minerals of a rock known as peridotite, which is common in the upper mantle where diamonds form.
Then the researchers used a piston cylinder to compress the small capsule and reach the pressures of diamond-forming zones, up to six gigapascals—akin to “a whole building standing on your foot,” Förster notes. Finally, the capsule was electrically heated to attain temperatures as high as 2,012 degrees Fahrenheit and then allowed to brew for between two and 14 days.
When the capsule's baking was complete, the researchers studied the results of the chemical reactions. They discovered a high ratio of potassium to sodium salts, similar to that found in the diamond inclusions, as well as the formation of a sodium-rich mineral known as clinopyroxene.
“It’s essentially this formation of clinopyroxene that soaks up the sodium in this reaction zone,” Stachel explains. That same reaction may be happening deep underground, allowing the diamond inclusions to achieve their curious chemistries.
The birth of a gem
Armed with this new information, the researchers now propose a tweaked model for how many of the world's known diamonds were born.
When a tectonic plate is shoved into the deep, they say, an earthy soup begins to form. Water is released as the minerals of the crust and clays from sediments are heated. The water becomes infused with dissolved carbon from organic material on the ocean floor, the mantle itself, or carbonate sands made from limestone and the skeletal remnants of ocean critters.
The newly proposed component, the melty ocean sediments, also get added to the mixture. This fluid then percolates up through the mantle, reacting with the surrounding rocks to produce the final carbon-rich, salty solution from which diamonds slowly crystallize.
Karen Smit, a diamond geologist at the Gemological Institute of America, is excited about the new work but cautions that there is still plenty to learn about how these gems form.
“This is happening in an inaccessible part of the Earth, and there’s just still a lot of unknowns out there,” she says.
Smit also emphasizes that not all diamonds form in the same way. Some diamonds seem to grow super-deep, at hundreds of miles below the surface. Some even contain bonus elements like boron that impart brilliant colors, as seen in the Hope Diamond's famous azure hue.
“Every day we see things that we don't understand,” Smit says, noting that her work at the GIA brings her into contact with hundreds of diamonds each day. Still, the new research is an exciting clue to add to our understanding of diamond formation—and it underscores an oft overlooked significance of these glimmering stones.
“Diamonds aren’t just pretty,” she says. “They also tell us a lot about the Earth.”
Related Topics
You May Also Like
Go Further
Animals
- Octopuses have a lot of secrets. Can you guess 8 of them?
- Animals
- Feature
Octopuses have a lot of secrets. Can you guess 8 of them? - This biologist and her rescue dog help protect bears in the AndesThis biologist and her rescue dog help protect bears in the Andes
- An octopus invited this writer into her tank—and her secret worldAn octopus invited this writer into her tank—and her secret world
- Peace-loving bonobos are more aggressive than we thoughtPeace-loving bonobos are more aggressive than we thought
Environment
- Listen to 30 years of climate change transformed into haunting musicListen to 30 years of climate change transformed into haunting music
- This ancient society tried to stop El Niño—with child sacrificeThis ancient society tried to stop El Niño—with child sacrifice
- U.S. plans to clean its drinking water. What does that mean?U.S. plans to clean its drinking water. What does that mean?
- Food systems: supporting the triangle of food security, Video Story
- Paid Content
Food systems: supporting the triangle of food security - Will we ever solve the mystery of the Mima mounds?Will we ever solve the mystery of the Mima mounds?
History & Culture
- Strange clues in a Maya temple reveal a fiery political dramaStrange clues in a Maya temple reveal a fiery political drama
- How technology is revealing secrets in these ancient scrollsHow technology is revealing secrets in these ancient scrolls
- Pilgrimages aren’t just spiritual anymore. They’re a workout.Pilgrimages aren’t just spiritual anymore. They’re a workout.
- This ancient society tried to stop El Niño—with child sacrificeThis ancient society tried to stop El Niño—with child sacrifice
- This ancient cure was just revived in a lab. Does it work?This ancient cure was just revived in a lab. Does it work?
Science
- The unexpected health benefits of Ozempic and MounjaroThe unexpected health benefits of Ozempic and Mounjaro
- Do you have an inner monologue? Here’s what it reveals about you.Do you have an inner monologue? Here’s what it reveals about you.
- Jupiter’s volcanic moon Io has been erupting for billions of yearsJupiter’s volcanic moon Io has been erupting for billions of years
- This 80-foot-long sea monster was the killer whale of its timeThis 80-foot-long sea monster was the killer whale of its time
Travel
- How to plan an epic summer trip to a national parkHow to plan an epic summer trip to a national park
- This town is the Alps' first European Capital of CultureThis town is the Alps' first European Capital of Culture
- This royal city lies in the shadow of Kuala LumpurThis royal city lies in the shadow of Kuala Lumpur
- This author tells the story of crypto-trading Mongolian nomadsThis author tells the story of crypto-trading Mongolian nomads